Biologically Response MRI Agents
Medical Imaging Agents Project
The aim of this program is to develop magnetic resonance (MR) molecular imaging strategies that will enable the in vivo monitoring of biological processes. Specifically we shall develop novel polymers for imaging of early markers of diseases such as melanoma and ovarian cancer, and the uptake and migration of dendritic cell-based vaccines into the lymph system.
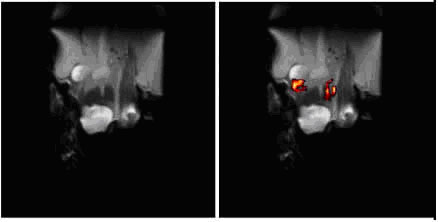
Figure 1. L: 1H density image of mouse post tail-injection with labelled dendritic cells
R: 19F image superimposed over proton density image
(Oliver Squires, PhD student of Prof Whittaker and Dr Blakey, University of Queensland)
A series of amphiphilic diblock copolymers of acrylic acid with partially-fluorinated acrylate and methacrylate monomers using ATRP as potential 19F MRI imaging agents have been synthesized. The diblock copolymers could undergo spontaneous self-assembly in mixed and aqueous solvents to form stable micelles of diameter from approximately 20-45 nm having a fluorine-rich core which provides a strong signal for MRI examinations.
It was found that the methacrylate polymers show systematically lower MRI signal intensity than acrylate polymers. As the solvent becomes more polar, the block copolymers are forced to assemble into micelles with a corona of polar poly(acrylic acid). Evidently the mobility of the fluorine-containing side chains becomes progressively more restricted, and the integrated intensities of the 19F signal of the trifluoroethyl side-chains decreases as solvent quality changes. The NMR properties of the block copolymer micelles have been demonstrated to depend strongly on the composition of the hydrophobic block. Specifically the flexibility of the backbone and the polymer-solvent interaction parameter determine the extent of molecular motion and hence averaging of the dipolar interactions between the nuclear spins. New generations of highly-branched and star copolymers are under investigation.
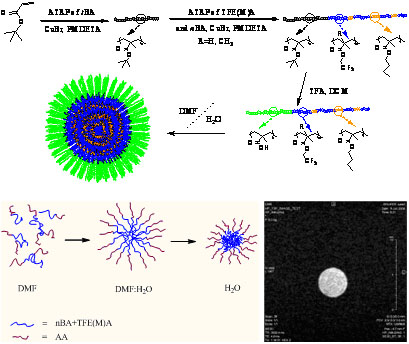
Figure 2. Synthetic scheme for preparation of amphiphilic diblock copolymers, the process of assembly and example MRI image.
Publications
- Peng, H.; Blakey, I.; Dargaville, B.; Rasoul, F.; Rose, S.; Whittaker, A. K. Biomacromolecules 2009, 10, 374.
- Thurecht, K.J.; Blakey, I.; Peng, H.; Squires, O.; Hsu, S.; Alexander, C.; Whittaker, A.K. Journal of the American Chemical Society 2010, 132, 5336.
- Nurmi, L.; Peng, H.; Seppälä, J.; Haddleton, D.M.; Blakey I.; Whittaker, A.K., Polymer Chemistry 2010, 1, 1039.
- Peng, H.; Thurecht, K.J.; Hsu, S.; Blakey, I.; Squires, O.; Kurniawan, N.; Rose, S.; Whittaker, A.K., ACS Symposium Series 2011, 1077(NMR Spectroscopy of Polymers), 459.
- Peng, H.; Thurecht, K.J.; Blakey, I.; Taran, E.; Whittaker, A.K., Macromolecules 2012, 45, 8681.
- Wang, K., Peng, H.; Thurecht, K.J.; Puttick S.; Whittaker, A.K., Polymer Chemistry 2013, 4, 4480.
- Wang, K., Peng, H.; Thurecht, K.J.; Puttick S.; Whittaker, A.K., Polymer Chemistry 2013, DOI: 10.1039/C3PY01311A.
- Yan K.; Li, H.; Wang, W.; Yi, C.; Zhang, Q.;Xu, Z.; Xu, H.; Whittaker, A.K., J. Mater. Chem. B 2013, DOI: 10.1039/C3TB21381A.
Collaborators
Prof Cameron Alexander, School of Pharmacy, University of Nottingham
Prof Dave Haddleton, School of Chemistry, University of Warwick
Prof Zushun Xu, Hubei University
Prof Stephen Rose, CSIRO
Source of Funding
Queensland Government Smart State NIRAP Grants, NHMRC Project Grants, ARC Australian Professorial Fellowship, ARC Discovery Grants, ARC Centre of Excellence
Point of contact: Hui Peng, Andrew Whittaker
